|

DANGER!
|
Highly Hazardous Chemicals Standard Operating Procedure
Pyrophoric and Water Reactive Materials
Including Organolithium reagents, Alkali metals, Metal Hydrides
Lab Specific Safety Information MUST be completed and approved
|

DANGER!
|
|
Hazards
|
Potential
Hazards
|
- H250: Catches fire spontaneously if exposed to air
- H260: In contact with water, releases flammable gases which may ignite spontaneously
- See SDS for specific product
- Improper handling and storage can result in fire or explosion leading to damage, personal injury, severe burns, or death. Any exposure to air or moisture will result in spontaneous ignition. Do not use highly hazardous chemicals (HHCs) in the laboratory unless there are no suitable alternatives.
- Pyrophoric: ignites spontaneously upon exposure to air at or below 54°C
- Water Reactive: reacts with water to produce flammable gases
|
|
Example chemicals:
- Alkali metals (e.g., lithium [7439-93-2], sodium [7440-23-5], potassium [7440-09-7])
- Metal hydrides (e.g., lithium aluminum hydride [16853-85-3], sodium hydride [7646-69-7], potassium hydride [7693-26-7])
- Organolithium reagents (e.g. n-butyllithium [109-72-8])
- Alkylaluminum reagents, (e.g. trimethylaluminum [75-24-1])
- Alkylzinc reagents (e.g. dimethyl zinc [544-97-8], diethylzinc [557-20-0])
- Boranes (e.g. tributylborane [122-56-5])
- Organophosphines (e.g. diphenylphosphine [829-85-6])
- Other organometallics (e.g. Grignard reagents)
- Trichlorosilane [10025-78-2]
- Finely powdered metals (e.g. zirconium)
- White phosphorous
|
|
Hazard Controls
|
Selection & Purchase
|
- Purchase only the smallest amount necessary for your work and discard old materials promptly.
- Select the lowest practical amount/concentration of pyrophoric/water reactive reagent.
- Use the less hazardous alternative whenever practical. I.e., sodium instead of potassium metal.
- Fire code limits the amount of pyrophoric and water reactive materials that can be stored on each floor of a laboratory building at any time.
- Use or properly dispose of old reagents before purchasing new materials to prevent exceeding the maximum allowable quantity (MAQ).
|
|
Storage & Transport – 1
|
- Store in the original compatible container with the manufacturer’s labels and warnings intact.
- Date upon receipt and opening of container.
- Store pyrophoric material away from heat/flames, oxidizers, flammable/combustible materials, water sources, and other incompatible materials listed in the SDS (e.g., acids, alcohols, oxygen).
- Store pyrophoric/water reactive materials upright in dedicated plastic or metal secondary containment in a flammable cabinet or explosion/fire-proof refrigerator per manufacturer’s recommendations.
- A glove box or dry box/desiccator may also be suitable.
- Pyrophoric/water reactive materials should be stored under an inert atmosphere of gas or oil compatible with the specific reagent.
- Alkali metals are generally stored under mineral oil, toluene, or kerosene.
- Metal hydrides and organometallics can be stored in a nitrogen or argon atmosphere or purchased as a dispersion in mineral oil. Lithium must be stored and handled under argon.
- Finely powdered metals are stored in a sealed container under an inert gas atmosphere.
- Butyllithiums are usually purchased in a solution of hexanes. Store under a blanket of inert gas.
|
|
Hazard Controls – 2
|
Storage & Transport – 2
|
- Reagents that are received in specialized packaging from the manufacturer (e.g., septa-sealed bottles such as Aldrich Sure/Seal, AcroSeal, etc.) should be stored per manufacturer guidelines.
- Reagents should be periodically examined to ensure containers and septa are in good condition.
- Ensure septa on septa-sealed bottles are in good condition prior to working with the reagent.
- Never return excess chemicals to the original container. If small amounts of impurities are introduced into the container, it may cause a fire or explosion.
- Transport in a bottle carrier or cart following guidelines set out in BU’s Chemical Hygiene Plan.
|
|
Engineering Controls & Safety Equipment
|
- All personnel must be aware of the locations of the emergency safety equipment prior to working with pyrophoric/water reactive chemicals.
 Safety shower and eyewash must be within 10 seconds travel of immediate work area without solid obstacles in direct path (i.e., doors) Safety shower and eyewash must be within 10 seconds travel of immediate work area without solid obstacles in direct path (i.e., doors)- Consult SDS for the correct fire extinguisher.
- Class D fire extinguisher should be used for alkali metals, zirconium, and metal hydrides fires.
 ABC extinguisher should be available for immediate emergency use. ABC extinguisher should be available for immediate emergency use.- Do NOT use water to attempt to extinguish pyrophoric/water reactive materials.
- Do not use CO2 fire extinguisher on an organolithium fire.
- Work MUST be performed in a chemical fume hood with the sash as low/closed as possible, or a glovebox with an inert atmosphere (e.g., nitrogen or argon).
- The addition of a portable safety shield is recommended when working in the hood and should be used when the potential exists for an explosion due to a high thermal reaction.
- An AtmosBag may also be used with highly controlled experiments, but it is not fire retardant.
- Do not use nitrogen as the inert gas when working with lithium metal.
|
|

|
|

|

|
|
Glovebox
|
Chemical fume hood
|
Portable shield
|
Atmosbag
|
|
Work Practice Controls – 1
|
- Complete “Lab Specific Information” page and submit for approval
- Carefully plan reactions, including quenching and disposal, minimizing energetic/unstable intermediates/biproducts. I.e., NaH in polar aprotic solvents.
- Do NOT scale up reactions using pyrophoric/water reactive reagents without PI’s prior approval.
- Do NOT work alone with highly hazardous chemicals.
- Work in sight/hearing of at least one other person familiar with this SOP.
- When working with highly pyrophoric materials, it is best practice to have a spotter with a fire extinguisher.
- Inform others in your laboratory before starting work with highly hazardous chemicals.
- Designate one area in the laboratory for pyrophoric use and prepare the environment accordingly.
- Remove clutter and flammable/combustible materials (including paper products) from the work area (hood/glovebox) prior to working with pyrophoric/water reactive materials.
- Keep a glass or metal container of sand in the workstation for covering small spills.
- All glassware must be completely free of moisture prior to working with these materials. Drying options are listed in order of preference:
- Oven-dried at least 110oC (overnight) – Cool in a desiccator, under vacuum, or an inert gas
- Heat gunned (5 minutes) under vacuum immediately prior to work
- Flame dried (30 seconds to 1 minute) under vacuum and inert gas immediately prior to work.
|
|
Hazard Controls – 3
|
Work Practice Controls – 2
|
- Preparing for solid pyrophoric/water reactive work
- Solid alkali metals should be cut and measured while submerged in a toluene or heptane bath.
- When weighing powdered water reactive materials on the benchtop, have container of sand or isopropanol at hand for spill clean-up.
- For highly reactive or easily spilled reagents, tare a clean, dry vial or round bottom, go to a hood to dispense an estimated amount of the solid, return to the balance and weigh reagent, returning to the hood to add or remove reagent until desired mass is achieved.
- Avoid removing mineral oil from powdered pyrophoric/water reactive chemicals prior to reaction.
 Preparing for liquid pyrophoric/water reactive work Preparing for liquid pyrophoric/water reactive work- Using a Schlenk line:
- Securely clamp the reaction apparatus and Schlenk line connected to a vacuum and inert gas source. When clamping apparatus, allow enough space under the reaction vessel for a raised lab jack holding the hot plate or ice bath.
- Nitrogen or argon are typically used as the inert gas with a Schlenk line.
- Do not use nitrogen when working with lithium metal.
 Cold traps can be cooled with dry ice and acetone (or liquid nitrogen) and must be regularly refilled. Cold traps can be cooled with dry ice and acetone (or liquid nitrogen) and must be regularly refilled.- Prepare the reaction vessel by attaching one Schlenk line hose and turning on the vacuum to remove air from apparatus.
- Dry glassware if necessary, by heat gunning or flame drying flask while under vacuum. Skip to step 6 if using oven-dried glassware.
- After 5 minutes (heat gun) or 1 minute (flame), turn the stopcock from the vacuum to the inert gas.
- Repeat flame dry/heat gun and back fill inert gas procedure twice more.
- Flow inert gas through the oil bubbler at approximately one bubble every two to three seconds to ensure positive pressure.
- Use a clean needle attached to the Schlenk line to puncture the reagent bottle’s septa. Needle should remain in headspace of bottle and inert gas must be flowing into bottle (stopcock open) prior to withdrawing reagent to equalize pressure.
- To avoid excess damage to the reagent bottle’s septa, use a 21-gauge or smaller needle (e.g., “green” disposable needle), on the Schlenk line hose.
- Choose syringe or cannula method of transferring reagent based on desired volume.
- An inert gas balloon is discouraged but may be necessary for very small volume work and can be done using a 15 mm thickness balloon.
- See Pyrophoric and Water Reactive Materials in the Teaching Lab SOP for detailed instructions.
- Choosing a syringe for dispensing <15 mL pyrophoric/water reactive liquids or solutions:
- Always use a Luer-lock or fixed needle syringe, never Luer-slip, when dispensing liquid reagents.
  Glass syringes with Teflon-lined plungers are best, disposable plastic syringes also seal well, avoid simple glass syringes when working with HHCs. Glass syringes with Teflon-lined plungers are best, disposable plastic syringes also seal well, avoid simple glass syringes when working with HHCs.- Use glass or all-plastic syringes with non-polar solvent containing chemicals (e.g., hexanes found in butyllithiums), as rubber plungers swell in organic solvents and become inoperable.
- Only fill the syringe to 50-60% of maximum capacity to reduce the risk of the plunger coming out of the back end of the barrel, causing an accident. I.e., 20 mL syringe for up to 12 mL of reagent.
- 16-, 18-, or 20-gauge needles long enough to reach below the bottle’s liquid level are recommended for use of pyrophoric chemicals.
|
|
Hazard Controls – 4
|
Work Practice Controls – 3
|
- Syringe dispensing steps:
- Prior to dispensing, securely clamp the reagent bottle inside the hood/glovebox.
- Insert needle connected to inert gas source into the headspace of reagent bottle.
- Flush dry syringe with inert gas from headspace three times. (Flushing a syringe, 5:13)
- Lower needle below liquid level and slowly draw up liquid reagent to avoid air bubbles and the risk of pulling plunger out of syringe.
- Inert gas should be flowing into the oil bubbler at roughly the same rate that reagent is pulled from bottle.
- Withdraw the desired volume of material.
- Remove syringe from bottle, then quickly transfer the material to the reaction apparatus.
- Be aware of potential liquid discharge, which may be caused by the over pressure in the bottle.
- Add the material by puncturing the rubber septum on the reaction flask with the needle or through an opening with gas counter flow.
- Inject pyrophoric/water reactive chemicals dropwise into the reaction flask.
- Remove Schlenk line needles from bottle septa when done withdrawing reagent.
- Upon addition of the pyrophoric/water reactive material, used needles and syringes must be immediately cleaned following the quenching steps outlined below.
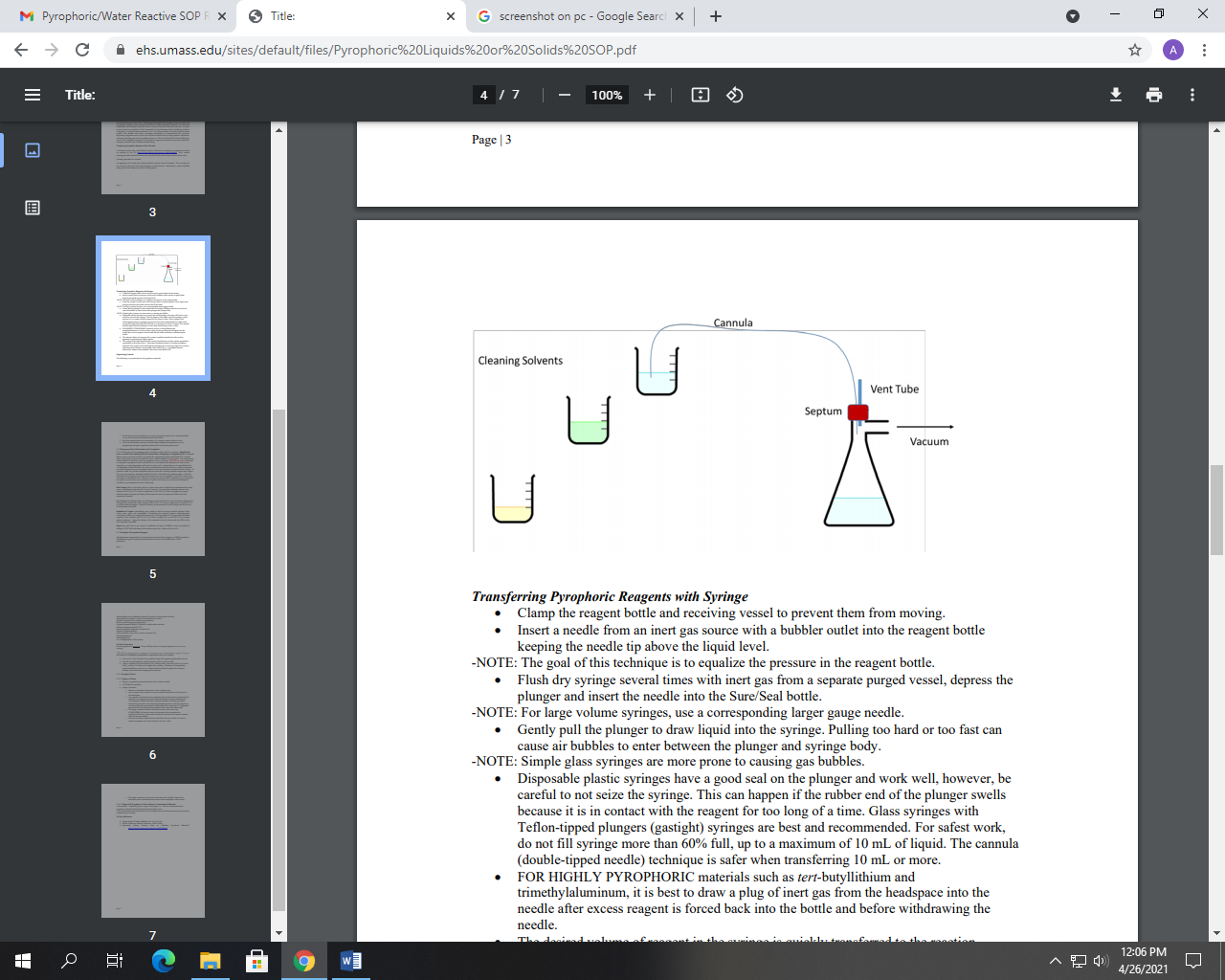
- Cannula/double-tipped needle dispensing steps:
- Ensure that reaction apparatus includes a dry calibrated addition funnel and a gas line to a bubbler.
 Prior to dispensing, securely clamp the reagent bottle inside the hood/glovebox. Prior to dispensing, securely clamp the reagent bottle inside the hood/glovebox.- Insert needle connected to inert gas source into the headspace of reagent bottle to pressurize the bottle.
- Insert one end of cannula through septa into the bottle’s headspace and flush the double tipped needle by allowing gas to flow through it.
- Insert other end of cannula through septa on top of calibrated addition funnel.
- Push the needle below the liquid level in the bottle while the inert gas is flowing. The positive pressure will force reagent through the cannula into the addition funnel connected to the reaction flask.
- Once the desired amount is transferred, pull the needle back up into the bottle’s headspace to stop the flow of liquid and to flush cannula with inert gas.
- First, remove the end from the reaction flask while making sure not to accidentally lower the needle tip below the liquid level in reagent bottle, then remove end from the reagent bottle.
- Remove Schlenk line needles from bottle septa when done withdrawing reagent.
- Slowly drip pyrophoric/water reactive reagent into reaction flask from addition funnel.
- Immediately upon addition of the material, clean the cannula by flushing it with toluene/heptane/hexane into an isopropanol bath following the quenching steps outlined below. (Cleaning double tipped needles, 8:31)
|
- Use of Glovebox for Pyrophoric/Water Reactive Liquids:
Hot glassware can be put into glovebox antechambers and transferred into the glovebox by three evacuation cycles (5 minutes) and purging with inert gas.
Opening of the antechamber to the outside can cause immediate fire when removing contaminated syringes, needles, wipes, glassware from the glovebox.
Best practice: bring an empty lidded glass container into the glovebox that can hold all the contaminated material and can subsequently be used to quench the material in the fume hood by filling with isopropanol, etc. If alcohol vapors are acceptable inside the glovebox, the initial quenching steps can occur in the glovebox prior to removing items.
|
|
Hazard Controls – 5
|
Work Practice Controls – 4
|
- General Quenching Tips:
- Any residual unused or unwanted reactive materials must be destroyed by hydrolysis and/or neutralization with adequate cooling. They should never be left open to the atmosphere.
- The quenching procedure should be performed in a well-ventilated fume hood.
- Remove all flammables/combustibles not needed for quenching from the area. The quenching of pyrophoric materials has caused a number of lab fires in fume hoods.
- Locate the proper fire extinguishers (type D extinguishers for alkali metals and metal hydrides) before starting.
- Never use water to initially quench the pyrophoric/water reactive material or a reaction where a pyrophoric reagent is used.
- Begin with a low reactivity quenching agent and slowly add more reactive quenching agents. Generally, while stirring, slowly add the following solvents in order:
|
1.
|
Toluene/Heptane/Hexane
|
bath (for solids) or rinse (for glassware and needles)
|
|
2.
|
Isopropanol
|
always
|
|
3.
|
Ethanol
|
as needed for large quantities
|
|
4.
|
Methanol
|
as needed for large quantities
|
|
5.
|
Water
|
always
|
|
6.
|
Citric acid (aqueous)
|
as needed
|
- After adding the solvents listed above, ensure a complete quench by stirring the mixture until visible reaction ceases, then neutralize as necessary. Even a small amount of unquenched material can react and result in a fire.
- Quench used syringes and cannula immediately after use, see below.
- Empty manufacturer’s bottles containing pyrophoric/water reactive residue should be treated as hazardous waste and do not need to be quenched. See Waste section below.
|
- To quench residual unspent pyrophoric material:
- Transfer unspent pyrophoric material to the appropriate reaction flask.
- Solid residue can be suspended in a bath of toluene or heptane flask. Work under an Ar atmosphere for Li metal.
- Liquid reagent can be transferred to a reaction flask under an inert atmosphere (N2 or Ar), using a bubbler to vent gases evolved during the quenching process. The inert atmosphere prevents solvents from catching fire if the mixture should become hot.
- Cool the flask to 0o C in an ice bath.
- While stirring, slowly add isopropanol to the reagent until it stops vigorously reacting.
- Slowly add progressively more reactive and polar solvents while stirring until the pyrophoric/ water reactive material appears to have been entirely consumed and the addition of water gives no sign of reaction.
- Isopropanol Ethanol Methanol Water
- Warm the mixture to 20o C and stir for an additional 15 minutes to overnight depending on the material and quantity to be quenched to ensure that the contents have completely reacted.
- As needed, neutralize the mixture with the addition of aqueous citric acid. The resulting hydroxides should dissolve, and the mixture should become homogeneous. Exercise extreme caution if the mixture is not homogenous at the end of this process, as small amounts of pyrophoric material may still be present; best practice is to stir the mixture until all solids dissolve.
- Once the process is complete, the mixture may be disposed of as organic solvent waste or in its own waste steam. See Waste section below for further details.
- Note: If a mercury amalgam is quenched, the resulting waste must be treated as mercury-contaminated waste.
|
|
Hazard Controls – 6
|
Work Practice Controls – 5
|
- To decontaminate equipment that have been used to handle pyrophoric materials:
- Glassware/equipment quenching steps:
- Rinse three times with hexanes followed by three isopropanol rinses to remove residue on equipment/glassware after contact with pyrophoric/water reactive materials.
- Next, place the materials in a large ice bath to quench and cool residue at the same time.
- Once the material is quenched, allow the ice bath to melt and stir the contents until it warms to 20o C.
-
-
- Only necessary for glassware or equipment that was not quenched during the reaction termination/chemical residue quenching procedure described above.
- Syringe/needle quenching steps:
- Draw hexane (or the solvent the pyrophoric material was in) into used syringe immediately after use, then inject into a beaker of isopropanol to dilute pyrophoric/water reactive residue. Flush with hexane a total of three times.
- Flush with isopropanol three times into the isopropanol bath.
 Cannula/double-tipped needle quenching steps: Cannula/double-tipped needle quenching steps:
- Fill a small flask with hexane and cover with a rubber septa. Pressurize the flask from the inert gas source.
- Insert the cannula into the hexane flask below the liquid level and allow the pressure to force hexane through the cannula into an isopropanol bath for at least 30 seconds.
- Repeat the previous two steps with isopropanol.
-
- If equipment/glassware cannot be conveniently rinsed, following one of the above procedures, small amounts of pyrophoric/water reactive material maybe quenched by directly placing it in a large ice bath to simultaneously cool and quench. Leave in ice bath until ice is completely melted. Never add flammable solvents if using this method.
|
|
Personal Protective Equipment
|
  Eye & Face Protection: Enclosed chemical splash goggles Eye & Face Protection: Enclosed chemical splash goggles
- Add a face shield when working outside of a glove box.
- NOTE: Safety glasses do NOT provide adequate protection from vapors.
- Hand Protection
- Disposable nitrile gloves of at least 5 MIL thickness are recommended for many common water reactive and/or pyrophoric chemicals. Consult product SDS for specific recommendations.
 Double gloves are recommended for working with highly pyrophoric chemicals outside of a glovebox. Double gloves are recommended for working with highly pyrophoric chemicals outside of a glovebox.
- Inner glove- Nomex flight gloves. Work with EHS to find appropriate brand.
- Outer glove- Disposable nitrile at least 5 MIL
- Remove any rings, watches, bracelets, etc. before working with HHCs.
- Inspect gloves for defects prior to use
 Skin and Body Protection Skin and Body Protection
 Fully closed lab coat – preferably Nomex or a flame-resistant cotton (i.e., Workrite™ FP or FR/CP) with snaps for ease of fast removal. Fully closed lab coat – preferably Nomex or a flame-resistant cotton (i.e., Workrite™ FP or FR/CP) with snaps for ease of fast removal.
- When working with large volumes of pyrophoric/water reactive material outside of a glove box, add a chemical resistant apron over the FR lab coat.
- Avoid wearing synthetic clothing
- Ankle length pants/skirt and wrist length long sleeves are required.
- Shoes must cover the entire foot.
- Liquid resistant shoe covers should be worn if shoes are not water repellent.
|
|
Emergencies & Spills
|
Medical Emergencies
|
- IMMEDIATE first aid and medical treatment are essential for people exposed to pyrophoric/ water reactive chemicals.
- People working with and around pyrophoric/water reactive materials should review SDS and other supporting safety documentation.
- All exposure to or contact with any pyrophoric/water reactive chemicals must receive immediate first aid and medical evaluation even if the injury appears minor.
- Call ROHP at 617-358-7647 in event of suspected pyrophoric/water reactive chemical exposure
- After treatment is sought, the PI must complete BU’s Occupational Injury/Illness Report Form for all lab related injuries and exposures within 24 hours of the incident.
FOR ACTUAL CHEMICAL EXPOSURE/INJURY
-
- Persons assisting an exposed colleague MUST wear PPE outlined above.
- IMMEDIATELY employ the safety shower/eyewash in the event of being splashed with pyrophoric/water reactive chemicals.
- Remove any contaminated clothing as quickly as possible while showering.
- Clothing should be cut off the body rather than being pulled over the head.
- Contaminated clothing must be bagged and disposed of as hazardous waste.
- Call Control 617-358-4444 (BUMC/BMC) or BUPD 617-353-2121 (CRC) to arrange medical treatment and transport while the victim is flushing the exposed area.
- Show SDS and pyrophoric first aid guidelines to medical provider
- Skin Exposure:
- Immediately drench skin in safety shower for at least 15 minutes to extinguish fire and lower body temperature.
- Eye Exposure:
- IMMEDIATELY flush eyes in the eyewash station for at least 15 minutes.
- WITHOUT interrupting the flushing, remove contacts and do not put them back in.
(Eyeglasses may be washed with soap and water and worn after decontamination.)
- Inhalation:
- Immediately move to fresh air.
- Ingestion:
- Do not induce vomiting. Seek immediate medical attention as severe and permanent damage to the gastrointestinal tract can occur.
|
|
Spills
|
- When a spill occurs, pyrophoric reagents will ignite immediately.
- Laboratory staff should not attempt to clean spilled pyrophoric material.
- Turn off all ignition sources if this can be done safely, vacate the area, and call for assistance.
- Minor spills can be smothered with sand then quenched with isopropanol.
- Call Control at 617-358-4144 (BUMC/BMC) or Facilities Emergency line at 617-353-2105 (CRC) and ask for EHS assistance before completing the spill clean-up.
- Follow the reporting procedures on the Emergency Response Card.
- Report the spill by calling Control at 617-358-4144 (BUMC/BMC) or Facilities Emergency line at 617-353-2105 (CRC).
- Ensure EHS is informed of all laboratory fires, as EHS must share this information with Boston Fire Department.
|


 Safety shower and eyewash must be within 10 seconds travel of immediate work area without solid obstacles in direct path (i.e., doors)
Safety shower and eyewash must be within 10 seconds travel of immediate work area without solid obstacles in direct path (i.e., doors) ABC extinguisher should be available for immediate emergency use.
ABC extinguisher should be available for immediate emergency use.



 Preparing for liquid pyrophoric/water reactive work
Preparing for liquid pyrophoric/water reactive work Cold traps can be cooled with dry ice and acetone (or liquid nitrogen) and must be regularly refilled.
Cold traps can be cooled with dry ice and acetone (or liquid nitrogen) and must be regularly refilled.
 Glass syringes with Teflon-lined plungers are best, disposable plastic syringes also seal well, avoid simple glass syringes when working with HHCs.
Glass syringes with Teflon-lined plungers are best, disposable plastic syringes also seal well, avoid simple glass syringes when working with HHCs. Prior to dispensing, securely clamp the reagent bottle inside the hood/glovebox.
Prior to dispensing, securely clamp the reagent bottle inside the hood/glovebox. Cannula/double-tipped needle quenching steps:
Cannula/double-tipped needle quenching steps:
 Eye & Face Protection: Enclosed chemical splash goggles
Eye & Face Protection: Enclosed chemical splash goggles
 Double gloves are recommended for working with highly pyrophoric chemicals outside of a glovebox.
Double gloves are recommended for working with highly pyrophoric chemicals outside of a glovebox.
 Skin and Body Protection
Skin and Body Protection
 Fully closed lab coat – preferably Nomex or a flame-resistant cotton (i.e., Workrite™ FP or FR/CP) with snaps for ease of fast removal.
Fully closed lab coat – preferably Nomex or a flame-resistant cotton (i.e., Workrite™ FP or FR/CP) with snaps for ease of fast removal.